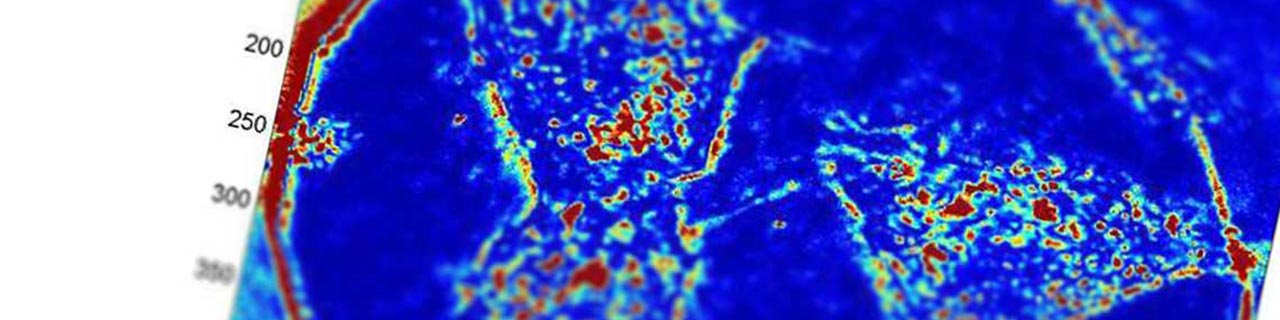
PWS NANOCYTOLOGY™ - Fixed
 We are focused on leveraging our PWS Nanocytology™ – Fixed Cell technology to develop low-cost screening tests that improve the early detection of cancer.
We are focused on leveraging our PWS Nanocytology™ – Fixed Cell technology to develop low-cost screening tests that improve the early detection of cancer.
We have validated the technology and its potential to detect cancer in more than 1,500 patients in seven different cancers (lung, colorectal, prostate, pancreas, ovarian, thyroid and esophageal).
Results have been comparable to current gold-standard screening technologies and we are preparing to initiate our pivotal clinical studies for the lung test in Q1 2018.
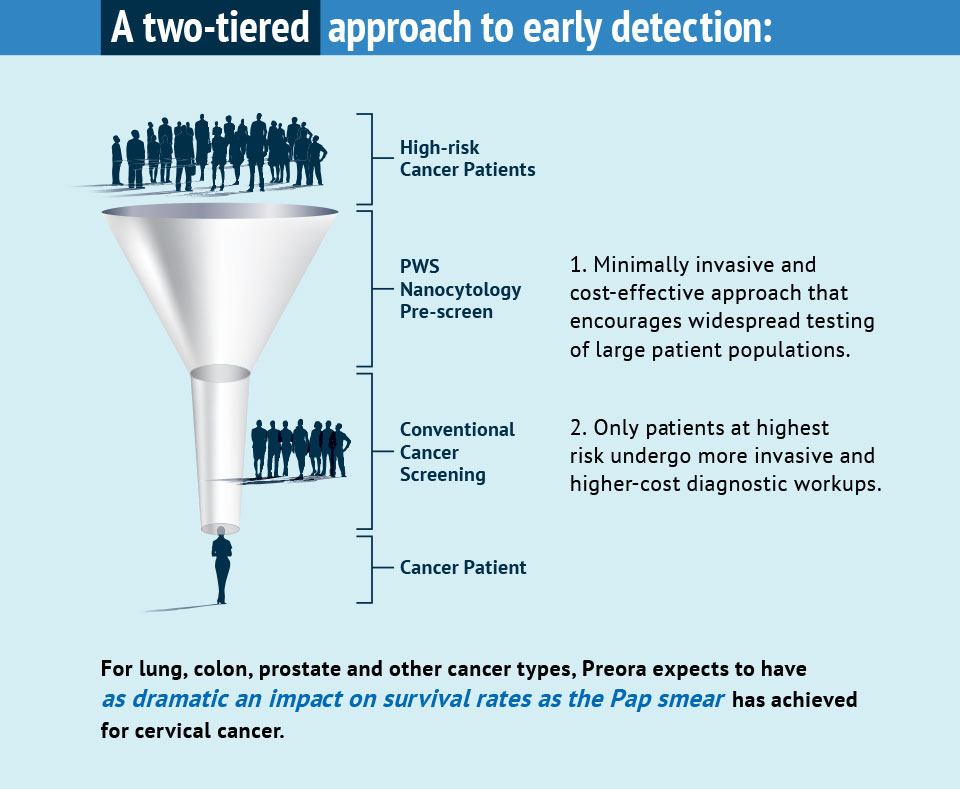
Market research indicates that low-cost, minimally invasive, highly accurate and easy-to-use screening tests, such as ours, enable physicians to identify patients most likely to benefit from more invasive, second-tier tests, such as Low-Dose Computed Tomography (LDCT) for lung cancer and colonoscopy for colorectal cancer.
An example of the impact of first-tier screening is the Pap smear; its widespread use has resulted in a 90 percent improvement in the survival rate for women with cervical cancer.
Cell samples for our tests will be collected using a simple brushing technique during routine physical exams in primary care physicians’ (PCPs) offices. Test results will enable PCPs to identify which high-risk patients warrant second-tier testing, and which can forego the costs and potential complications of more invasive cancer screening. We anticipate high adoption rates of our tests in this multibillion-dollar market.
Initial Market Opportunities
 Lung Test: Approximately 12 million Americans are at high risk for lung cancer (ages 55-80 with a history of smoking). Millions more have hereditary concerns and/or have been exposed to potentially carcinogenic environmental conditions. We estimate the total domestic market size at approximately $2 billion/year. International markets with high smoking and environmental risks also provide significant revenue opportunities.
Lung Test: Approximately 12 million Americans are at high risk for lung cancer (ages 55-80 with a history of smoking). Millions more have hereditary concerns and/or have been exposed to potentially carcinogenic environmental conditions. We estimate the total domestic market size at approximately $2 billion/year. International markets with high smoking and environmental risks also provide significant revenue opportunities.
Colorectal Test: Approximately 80 million Americans are at risk for colorectal cancer (ages 50 – 75). Only 50% of those at high risk are currently being tested and a vast majority of those tests confirm that a patient does not have polyps/advanced adenomas. The potential for a low-cost, minimally invasive, highly accurate and easy-to-use test, such as ours, has significant potential in the current $12 billion/year market. Our test is designed to identify patients at high risk for developing colorectal cancer, reserving invasive colonoscopies for patients most likely to benefit from the procedure.